Overview of IIDP Study Design
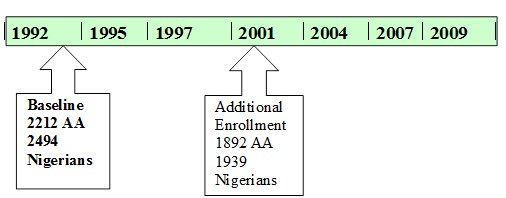
The IIDP has recruited 8000+ individuals since 1991 and conducted longitudinal evaluations every 2 to 3 years with up to 7 evaluations per individual. Figure 1 illustrates the study timeline. Recruitment to the study was conducted at two time points. In the first recruitment in 1992, community dwelling African Americans age 65 or older living in Indianapolis (n=2212) and community dwelling Yoruba age 65 or older living in Ibadan, Nigeria (n=2486), were recruited to the study. In 2001, the IIDP conducted another wave of recruitment in both populations. The age cut-off for the 2001 cohorts was chosen in order to maintain comparability with the 1992 cohort since the youngest participants in the 1992 cohort had since turned 70; In Indianapolis, community-dwelling subjects were randomly selected from Medicare records, who identified themselves as African-Americans, and were at least 70 years of age. 1,892 African Americans were recruited during the 2001 evaluation wave. In Ibadan, a house-to-house census was conducted and 1,939 community dwelling aged 70 years and older Yoruba were recruited during the 2001 wave.
Figure 1. IIDP enrollment and evaluation timeline.
All participants agreed to undergo regular follow-up cognitive assessment and clinical evaluations. The study was approved by the Institutional Review Boards of Indiana University-Purdue University of Indianapolis and University of Ibadan. All participants provided informed consent. Details on the assembling of the original cohorts and the enrichment cohort were described in Hendrie et al (Am J Psychiatry, 1995) and Hendrie et al (JAMA, 2001).
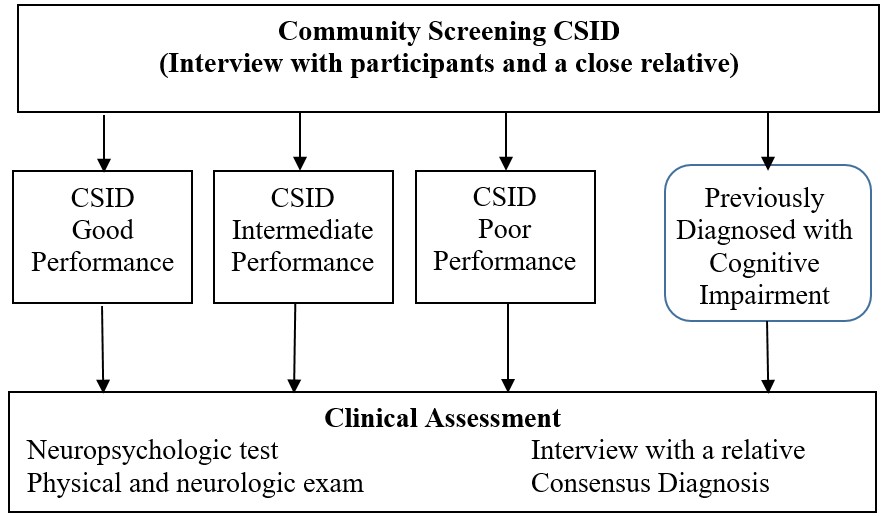
At each evaluation, a two-stage sampling design was used where all participants were first evaluated with a brief harmonized cognitive instrument and interview from a close relative. Figure 2 illustrates the two-stage design used for each wave. Participants were stratified based on results from the first stage to determine their probabilities of dementia, then randomly selected within each stratum to receive extensive neuropsychological tests and clinical evaluations for the diagnosis of dementia, AD, mild cognitive impairment (MCI) or normal cognition. Please see file ??? in Study Documents for cut-off points used for defining screening groups for each wave of the study. At the first stage, extensive information on demographic, lifestyle, medical history and function were also collected. In the course of longitudinal follow-up, information on social, cognitive and physical activities, depressive symptoms, and anxiety as well as neurological examinations, were added to the first stage evaluation at various times.
IIDP Data Components
Data Collection Forms
Since the beginning of IIDP in 1991, many aspects of the data collection and database evolved. Data collection forms for each wave of evaluation expanded to include more items to ascertain potential risk factors and to evaluate emotional health as well as to conduct neurological and physical exams
At each of the 7 evaluation waves prior to 2011, two data collection packets were used: one for the screening phase consists of interviews with study participants and interviews with a close relative of the study participant; a second data collection packet was for those selected for clinical evaluation at the 2nd phase. For each data collection packet, an English version was used for Indianapolis, and a Yoruba version (with English subtext) was used in Ibadan. In 2011, a screening phase was conducted of surviving participants, but no clinical assessment was conducted due to the end of funding.
Figure 2. Two-stage design with screening and clinical evaluation.
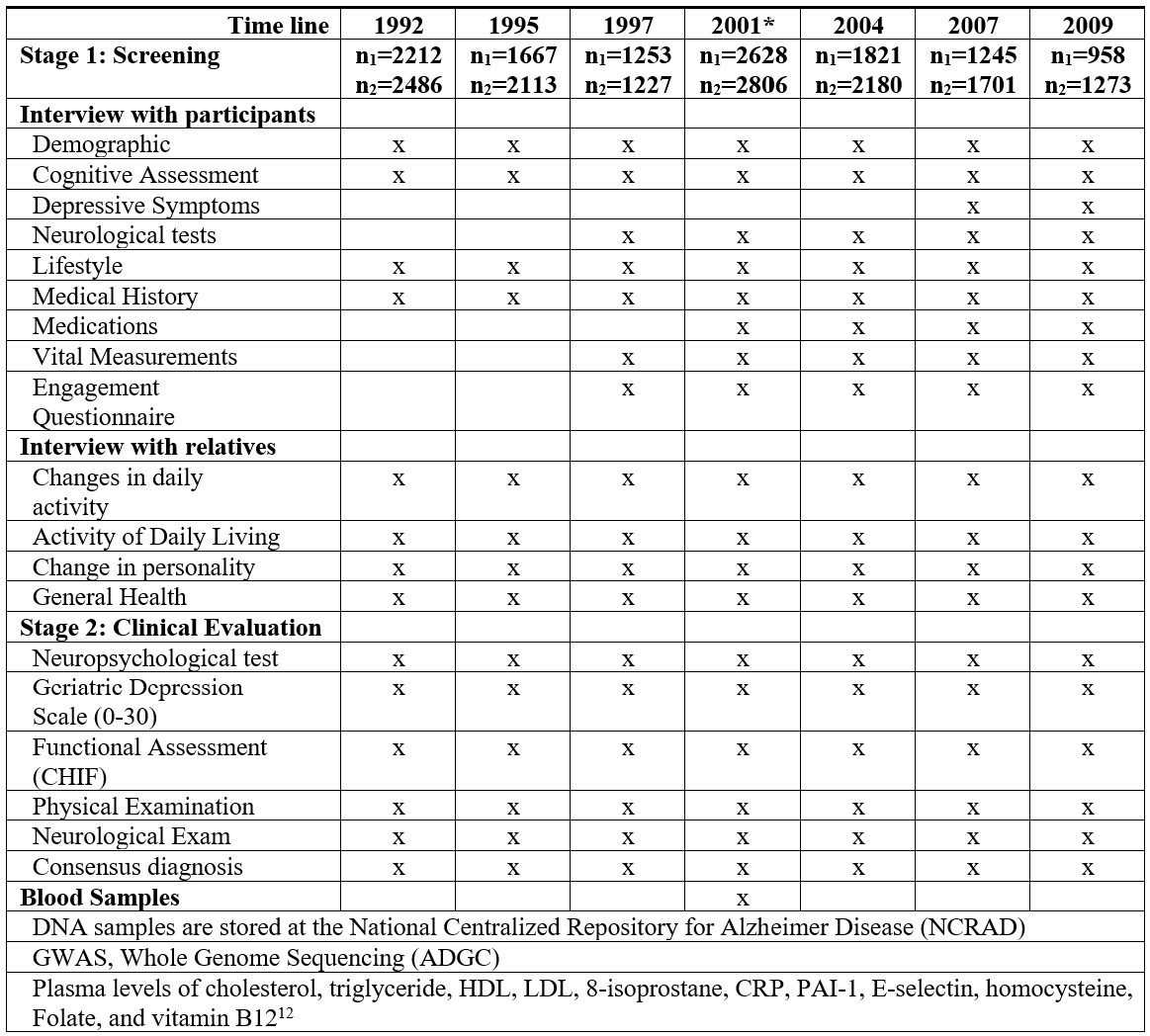
Demographic information includes age, gender, education, marital status, living arrangement, primary occupation, urban or rural residence until age 19, and family history of dementia.
Cognitive Assessment The Community Screening Interview for Dementia (CSID) was developed by our group specifically for use in comparative epidemiological studies of dementia in disparate populations with various cultural backgrounds and literacy levels (Hall et al, Int J Geriatr Psychiatry. 2000) The CSID consists of two parts: an interview with the study participant measuring cognitive function, and an interview with a close relative to gather information on daily functioning and cognitive decline. Cognitive assessment items were selected to measure the following functions in a short interview: memory, abstract thinking, judgment, and other disturbances of higher cortical function (aphasia, apraxia, agnosia, and constructional difficulty), personality changes and daily functioning at work, home and in social relationships. Two scoring algorithms have been used for the CSID: the original scoring for dementia screening, and a revised scoring allowing sub-domain scores for memory, semantic knowledge and executive function.
Depressive Symptoms were collected using the 15-item Geriatric Depression Scale (GDS) starting in stage 1 at the 2004 evaluation. Note that the 30-item GDS was used at the stage 2 clinical assessment.
Lifestyle questions included alcohol consumption and smoking habits. The questions on smoking include whether current, former or non-smoker and the frequency and quantity smoked if applicable.
Physical measurements and vital signs include weight, height, waist circumference, and blood pressure measurement (two times). Self-reported Medical history includes hypertension, diabetes, cancer, stroke, heart disease, head injury, and fracture. If any chronic condition was reported by either a study participant or his/her informant, we inquired the approximate time of on-set.
The engagement questionnaire section included questions of whether the subject was caring for others, the relationship between the subject and the one being cared for, whether the subject visited family/friends regularly, and if he/she participated in local events and chores. There were also various questions about activities were tailored to each site.
Neuropsychological tests used at stage 2 clinical evaluation include the Mini-Mental State Examination (MMSE), Animal Fluency Test, Boston Naming, CERAD Word List (learning and recall), Construction Praxis, and IU Token Test.
Functional Assessment (CHIF): A 10 item semi-structured home-based interview to assess function by measuring instrumental activities of daily living directly (Hendrie et al Int Psychogeriatr. 2006).
Consensus Diagnosis: For participants with clinical assessment, diagnosis of dementia, cognitive impairment or normal was made in consensus diagnosis across the two sites. Subtypes of dementia including Alzheimer’s Disease were also given. Details on diagnosis criteria and process were described in Hendrie et al JAMA 2001.
Blood Samples and Biomarkers Blood samples from 1,510 African Americans and 1,254 Yoruba participants were collected at the 2001 evaluation wave. Blood samples were drawn in 10-mL EDTA Vacutainer tubes. The specimens were transported on ice from the field to the laboratory at Indiana University School of Medicine for the African American cohort, or at Ibadan University College Hospital for the Yoruba samples. In the laboratory, erythrocytes, buffy coat, and plasma were separated. After labeling the plasma and buffy coat tubes with a unique bar code for each participant, the samples were stored in a −70°C freezer. Samples were shipped to Indiana University in approved blood shipping containers with dry ice and arrived usually within three days. Samples from Indianapolis were directly processed at Indiana University. Biochemical analyses were carried out on the plasma.
Cholesterol, triglyceride, and high-density lipoprotein (HDL) cholesterol levels were determined by using commercial kits from Roche Diagnostics (Indianapolis, Indiana). Low-density lipoprotein (LDL) cholesterol levels were calculated by using the Friedewald equation. PAI-1, E-selectin, 8-isoprostane, homocysteine, folate, vitamin B12, and C-reactive protein were determined by using commercial kits from BioRad, Hercules, Calif; Cayman, Ann Arbor, Mich; and Diasorin, Stillwater, Minn.
DNA Samples DNA samples have been collected using filter cards in early waves of the study and later from blood samples. DNA samples for both African Americans and Yoruba cohorts are stored at National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD) and these samples have been shared with various GWAS or WGS consortium groups.

